Contributing to less than 200,000 cases per year in the US, retinitis pigmentosa (RP) is a type of retinal dystrophy that results in blindness from a loss of function in the retina.
There is no cure for this condition yet, just the treatment of its symptoms. Like RP, there are several other inherited retinal diseases like Leber congenital amaurosis (LCA), which requires the attention of researchers and drug manufacturers.
In December 2017, a pharmaceutical company called Spark Therapeutics launched a breakthrough one-time gene therapy, LUXTURNA™ (voretigene neparvovec-rzyl), for patients with biallelic RPE65 (retinal pigment epithelial 65 kDa protein) mutation-associated retinal dystrophy.
Alex Levin of the Wills Eye Pediatric Ophthalmology and Ocular Genetics service, said: “FDA approval of LUXTURNA represents a true paradigm shift for physicians caring for patients with hereditary retinal disease caused by biallelic RPE65 mutations, who up until now have had no pharmacologic treatment options.”
Novartis Acquires Rights To Drug Outside US Markets
Earlier this year, Novartis announced a licensing agreement with Spark Therapeutics for the development and commercialization of FDA-approved voretigene neparvovec-rzyl, LUXTURNA™, outside US markets.
Currently, there is no therapy for this set of diseases outside the United States. Shreeram Aradhye, Head of Medical Affairs Novartis Pharmaceuticals, explained: “No otherwise healthy child should have to go blind due to this devastating disease. Gene therapy is a promising new avenue to potentially address this unmet need.” He also said that this collaboration will ensure a global presence and enable patients outside the US to access this “potentially life-changing treatment.”
Spark will release the treatment in the US, in March, at a price of $850,000. The company will be entitled to royalties in the future, apart from the $100 million from Novartis and $65 million following the European regulatory approval and initial sales.
As expert analyst, Micheal Yee, mentioned to future investors, this collaboration will allow Spark to concentrate its efforts on the US, while Novartis, a pioneer in the ophthalmology domain, could take over the commercialization of the drug in markets outside the country.
What Is LUXTURNA & What Does It Do?
Luxturna™, the adeno-associated virus (AAV) vector-based gene therapy, is indicated for patients diagnosed with biallelic RPE65 mutation-associated retinal dystrophy. The condition is caused by mutations in the RPE65 gene of the retinal pigment, thus resulting in blindness or impairment of vision.
Successful clinical trials were conducted on 41 test subjects — including children above the age of 15 and adults — with positive biallelic RPE65 mutation-associated retinal dystrophy. The drug, i.e., a viral vector containing the corrected gene, was injected into patients’ subretinal space, and this helped reactivate the pigment, thus restoring vision.
UPenn’s Prof. Jean Bennett voiced his opinion about this ophthalmological drug, and said: “During the more than 12 years of innovative research with dedicated collaborators near and far, I’ve witnessed the dramatic improvement in vision in many patients who would have otherwise lost their sight. I believe that the success of the LUXTURNA™ clinical development program will pave the way for the development of other gene therapies, that may help the millions of patients with genetic diseases who currently have limited or no treatment options.”
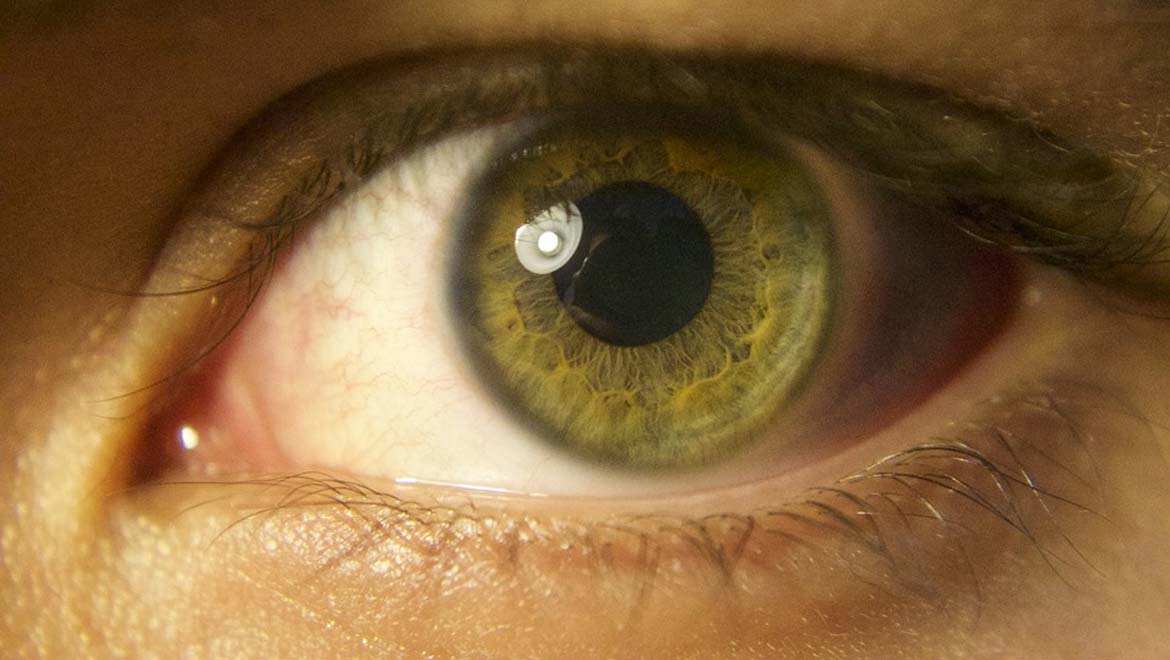
Top image: Retina (Public Domain)
References:
National Eye Institute (NEI), https://nei.nih.gov/health/pigmentosa/pigmentosa_facts, (accessed 14 Feb 2018)
Novartis (2018), https://www.novartis.com/news/media-releases/novartis-exclusively-licenses-first-ophthalmology-gene-therapy-all-markets-outside-us-milestone-patients-rare-inherited-vision-loss, (accessed 15 Feb 2018)
Reuters (2018), https://www.reuters.com/article/spark-luxturna-novartis/spark-licenses-blindness-gene-therapy-rights-outside-u-s-to-novartis-idUSL2N1PJ2EE, (accessed 15 Feb 2018)
Spark Therapeutics (2018), http://ir.sparktx.com/news-releases/news-release-details/fda-approves-spark-therapeutics-luxturnatm-voretigene-neparvovec, (accessed 15 Feb 2018)
Beasley, D. (2018), Reuters, https://www.reuters.com/article/us-spark-luxturna-novartis/spark-licenses-blindness-gene-therapy-rights-outside-u-s-to-novartis-idUSKBN1FD35R, (accessed 16 Feb 2018)





No comment