The accumulation of discarded plastics is a global issue, particularly as it exists in the same landfills for hundreds of years. This makes it a prevalent health problem, as plastics can find their way into the food chain and vital ecosystems, thereby reducing the quality of the food and water we consume.
However, biochemical and bioengineering researchers believe that their work can help reduce the global plastic burden by the improved ability to decompose them.
For example, a recent joint project conducted at the University of Portsmouth, the United States' National Renewable Energy Laboratory (NREL), the University of South Florida and the University of Campinas (Brazil), has resulted in an enzyme that may be able to do away with polyethylene terephthalate (PET). This work will also be documented in an upcoming issue of the journal, PNAS.
PETase – The Enzyme Which Can Degrade Plastic
The project was originally set up to investigate the evolutionary responses to the presence of plastics in nature.
PETase is one such enzyme that 'digests' this popular form of plastic. It was found to be present in the novel bacterial species, Ideonella sakaiensis, originally discovered in a recycling facility. Enzyme PETase was seen to be selectively specific for the plastic and degrades it with considerable efficacy too.
Lead researchers of this investigation, John McGeehan (Portsmouth) and Gregg Beckham (NREL), initially set out to conduct an advanced crystallography study of PETase. The experts wanted to define its three-dimensional structure and plastic-eating mechanism, conclusively. But, the project ended up taking different turns, which went beyond the scope of their original goal.

John McGeehan, the joint lead investigator of the project. (Source: Portsmouth University)
The study also included steps to replicate the PETase structure. These measures included inducing variations in the gene for the enzyme to see the parts of it related to the protein's function.
With this, the researchers realized that the newly engineered mutant PETase was more efficient in terms of plastic degradation than the original enzyme. Their version of this protein molecule could also digest polyethylene furandicarboxylate (PEF), a material that is currently being promoted as "bio-based" PET and used in bottles and other packaging.
The researchers have regarded this breakthrough as a potential boon and the future of plastic recycling.
John McGeehan, a professor of biochemistry at Portsmouth, notes that the improved PETase could be integrated into processes that break up PET and PEF for recycling.
In addition, the researchers were successful in their aim to understand the PETase structure in greater detail.
Reports suggest that the enzyme could be homologous to cutinase. This small protein, originally discovered in fungi, hydrolyzes cutin, a polymer found in plants. Cutinase has also exhibited a considerable amount of versatility and is used in a diverse range of industrial applications today.
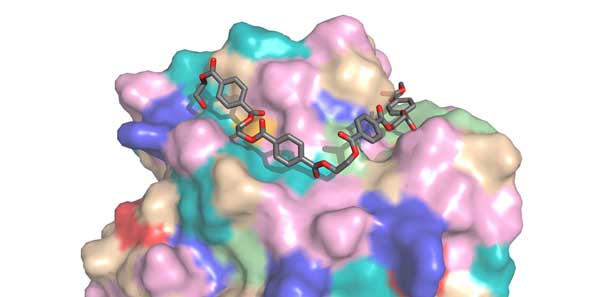
The PETase active site, complete with a molecule of PET (Source: H Lee Woodcock, University of South Florida)
The structural part of the study was completed using a powerful X ray-powered microscope located at the Diamond Light facility in the UK. The instrument was able to produce 3D images of PETase at a high resolution, encompassing its conformation at the atomic level and advanced information on its active site. The data helped researchers understand PET-degrading functions better, and eventually, led to improvements on the newly-developed enzyme.
Computational modeling completed at Campinas and South Florida led to further details on how PETase differed from cutinase. For instance, PETase was found to have a different catalytic site in order to be compatible with the artificial polymer.
The researchers, thus, decided to mutate the PETase gene to alter this active site in the protein that resulted in the new, superior version.
It has now been concluded, by experts, that bacteria such as I. sakaiensis may have adapted to produce PETase as a response to life, in high-PET environments. The bacteria are known to perform such activity in order to derive energy and carbon from the plastic.
This process also converts the plastic into its 'eco-friendly' precursors - ethylene glycol and terephthalic acid. Therefore, this new form of PETase may be the key to reducing the current global plastic burden in the future.
Top Image: PET is a plastic that can contribute to pollution and dumping, worldwide. (Source: Public Domain)





No comment