This week, scientists managed to modify human embryos to remove a genetic mutation which causes heart failure in young people. A mutation of the MYBPC3 gene causes hypertrophic cardiomyopathy, or HCM.
As noted in the study, HCM is “a myocardial disease characterized by left ventricular hypertrophy, myofibrillar disarray and myocardial stiffness; it has an estimated prevalence of 1:500 in adults and manifests clinically with heart failure. HCM is the commonest cause of sudden death in otherwise healthy young athletes. HCM, while not a uniformly fatal condition, has a tremendous impact on the lives of individuals, including physiological (heart failure and arrhythmias), psychological (limited activity and fear of sudden death), and genealogical concerns. MYBPC3 mutations account for approximately 40% of all genetic defects causing HCM and are also responsible for a large fraction of other inherited cardiomyopathies, including dilated cardiomyopathy and left ventricular non-compaction.”
Current strategies for treating HCM
At the moment, treatment options for HCM are limited to providing relief from symptoms, as opposed to addressing the genetic background of the disease. Therefore, it was decided to see if it could be possible to develop a new technique through which it might be possible to correct the possibility of the mutation being able to express itself.
The scientists first looked for a male carrying a single copy of the mutated MYBPC3 gene, before making embryos using eggs from healthy volunteers without the mutated gene. Half the embryos were found to carry the gene mutation, as expected. If these embryos were allowed to grow to full term, the child born would carry the faulty gene and as a result, inherit the HCM heart condition.
In order to achieve their goal, the scientists involved in this study employed a technique of ‘editing’ genes, known as Crispr-Cas9. When this was carried out at the same time as fertilization, the team managed to achieve 42 healthy embryos out of a total of 58. In other words, 72 percent of the embryos were free of the deadly mutation.
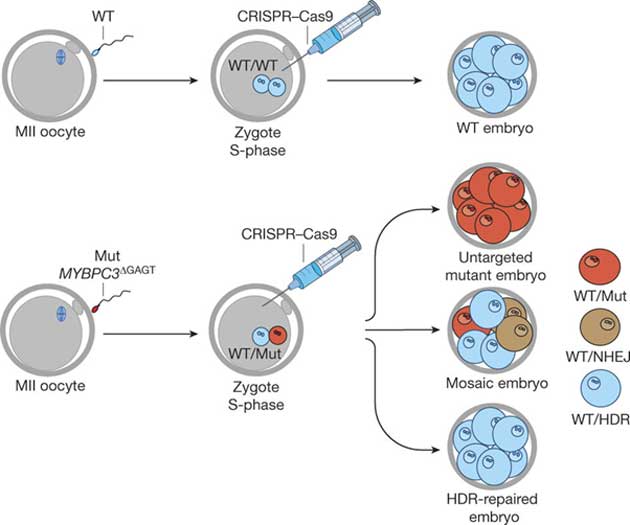
Schematic of MYBPC3∆GAGT gene targeting by injection of CRISPR–Cas9 into human zygotes at the S-phase of the cell cycle. MII oocytes were fertilized by sperm from a heterozygous patient with equal numbers of mutant and wild-type (WT) spermatozoa. CRISPR–Cas9 was then injected into one-cell zygotes. Embryos at the 4–8-cell stage were collected for genetic analysis. Injection during S-phase resulted in mosaic embryos consisting of non-targeted mutant, targeted NHEJ-repaired and targeted HDR-repaired blastomeres. (Nature)
Next steps
While this step forward for medical research definitely won’t result in any genetically modified babies, it does increase the potential for clinical trials on humans to be approved. The next step is to see whether or not the technique also works for mutations that are carried by the egg as opposed to the sperm. The paper, titled ‘Correction of a pathogenic gene mutation in human embryos’ was published online in the journal Nature, this week.
Many people worry about advances in gene editing such as these eventually being used to produce designer babies. Janet Rossant, a co-author of the US National Academy of Sciences report on gene editing, said it was a distant prospect. “We are still a long way from serious consideration of using gene editing to enhance traits in babies. We don’t understand the genetic basis of many of the human traits that might be targets for enhancement. Even if we did, a genetic alteration that enhanced one trait could have unexpected negative consequences on other traits, and this would be an inherited feature for the next generation. The NAS report came out strongly against any form of gene editing designed to simply enhance human potential.”
Despite this, this technique certainly shows the potential of gene editing and how the technique is becoming more advanced. As geneticist Richard Hynes said of the study: “This brings it closer to the clinic, but there’s still a lot of work to do.”
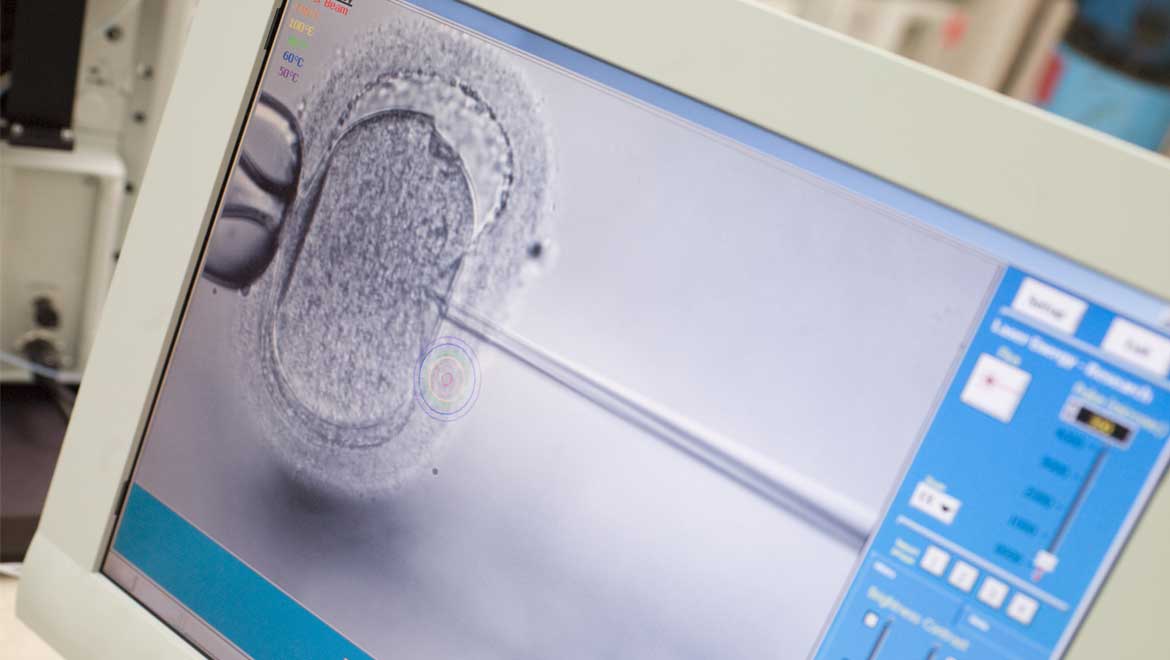
Top image: Monitor showing intra cytoplasmic sperm injection (ICSI) (Graphicstock)




No comment