In ancient times and up until the late 1800s a popular theory was that infectious disease was caused by miasmas, foul vapors emitted by rotting material. Swamps were a particularly strong source of “bad air;” in Italian this was referred to as “mala aria” and the disease caused by vaporous swamps came to be known as “malaria.” In fact, although we didn’t know it, we were engaged in genetic warfare against the true cause of malaria, the Plasmodium parasite carried by mosquitoes that thrive in swamps. Recent research, by Ellen M. Leffler and colleagues, describes a new front in this ongoing battle.
The Impact of Malaria
Malaria infects more than 350 million people with more than 1 million people dying from it each year. The Plasmodium parasite is a single-celled organism with a complex life cycle. It reproduces sexually in the Anopheles mosquito but also must make its way into humans to reproduce without sex. Humans serve as a reservoir for infecting new mosquitoes. While it resides in humans a critical step in its maturation requires it to invade red blood cells. When Plasmodium parasites are ready to move on to the next mosquito they cause their host red blood cell to burst open. This rupture leads to the release of toxins that can cause fevers, seizures, and death. The Plasmodium times its break-out based on the slight drop in body temperature that occurs as we sleep at night. This is also when mosquitoes are most active. Historically, the most effective measures against malaria have been those taken against the Anopheles mosquito, including draining swamps, spraying insecticide, and the use of mosquito nets.
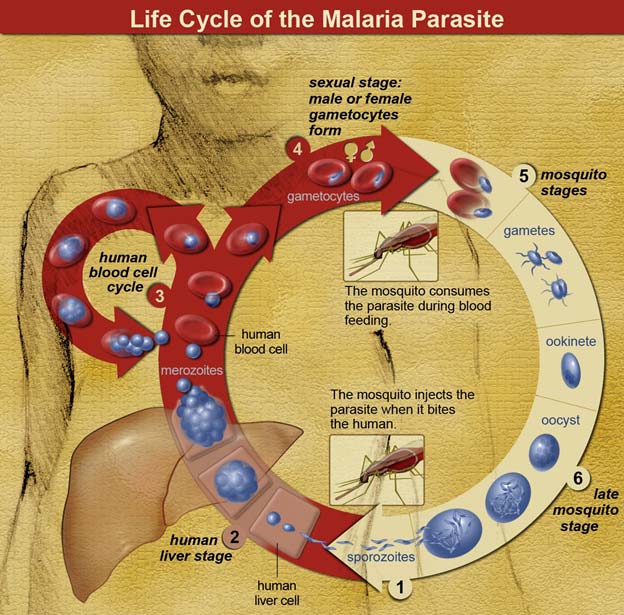
The life cycle of malaria parasites. A mosquito causes an infection by a bite. First, sporozoites enter the bloodstream, and migrate to the liver. They infect liver cells, where they multiply into merozoites, rupture the liver cells, and return to the bloodstream. The merozoites infect red blood cells, where they develop into ring forms, trophozoites and schizonts that in turn produce further merozoites. Sexual forms are also produced, which, if taken up by a mosquito, will infect the insect and continue the life cycle. (Public Domain)
The Human Response
In warm and humid climates where the Anopheles mosquito lives, natural selection apparently favors human populations that have developed changes in their genes that inhibit the growth of the Plasmodium. One famous example is sickle cell hemoglobin. Hemoglobin is the protein that carries oxygen in red blood cells. Like almost all genes, everyone gets one copy of this gene from each parent. If a person receives two copies of the sickle cell hemoglobin gene they suffer from sickle cell anemia. If they only receive one copy they are said to be carriers. Carriers have enough normal hemoglobin to be healthy, and yet their red blood cells are fragile enough to inhibit the reproduction of the Plasmodium. People with one copy of the sickle cell hemoglobin gene generally have less severe cases of malaria. About 4.4 million people have two copies of the sickle cell gene, while about 43 million people have only one copy. The prevalence of sickle cell roughly corresponds to the historic distribution of malaria.
This is a good illustration of the sometimes ruthless actions of natural selection. There is absolutely no advantage to an individual in having two copies of the sickle cell hemoglobin gene. In fact, their weakened condition is more susceptible to malarial symptoms.
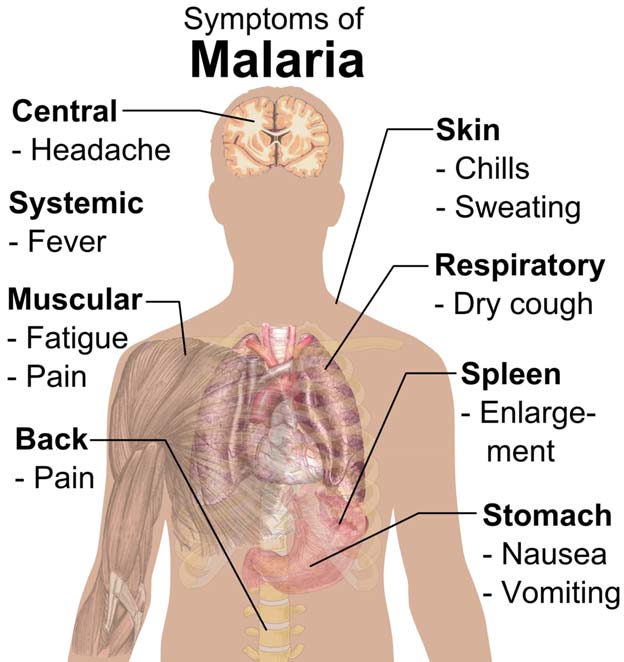
Main symptoms of malaria (Public Domain)
However, the population as a whole benefits from the presence of the much larger number of individuals with only one copy of the allele. The way genetics works, a couple in which both partners have one copy of the sickle cell gene have a 50% chance of having a child with one copy of the sickle cell gene (healthy and malaria resistant), a 25% chance of having a child with no sickle cell gene (healthy but vulnerable to malaria), and a 25% chance of having a child with two sickle cell anemia genes (suffering from sickle cell and vulnerable to malaria). If only one parent is a carrier of sickle cell there is also a 50% chance that the children will be carriers (healthy and protected) and none of the children will suffer from sickle cell. So, in response to the onslaught of malaria evolution produces many protected individuals by a mechanism that ensures that other individuals will be unprotected from malaria and many individuals will suffer from sickle cell.
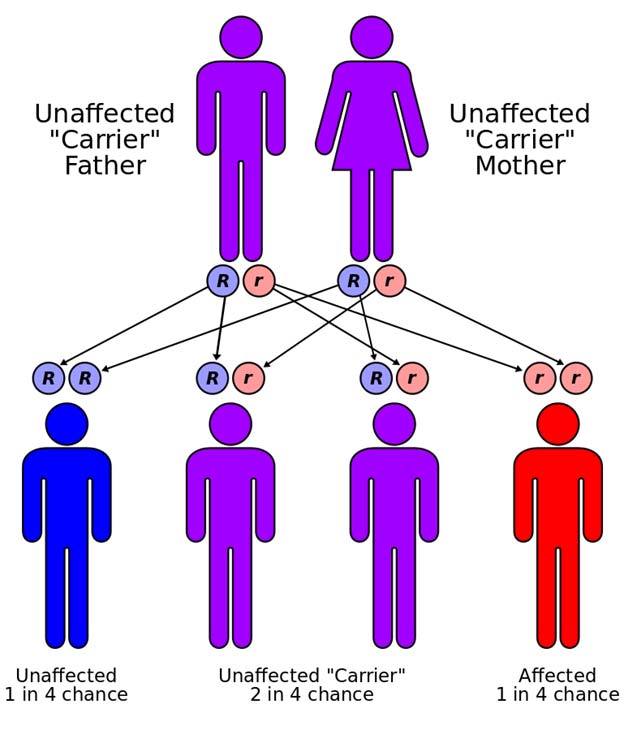
Autosomal recessive gene (CC BY-SA 3.0)
The Surface of Red Blood Cells
Natural Selection has found other ways to protect humans against Plasmodium. Populations that are under Plasmodium attack often have different kinds of proteins on the surface of their red blood cells. Plasmodium needs to bind to proteins on the surface of red blood cells in order to invade them, although it seems to be flexible about what proteins it will use. There are several different proteins of which it might take advantage. For example there is a protein, the Duffy Antigen, which immature red blood cells seem to need to respond to hormones and is retained on mature red blood cells in most people. However, it is missing on the mature red blood cells of most West Africans, and the Plasmodium that is dependent on the presence of this protein, Plasmodium vivax, is also missing in West Africa. So, it would appear that humans won that particular skirmish.
Other forms of Plasmodium use other proteins to invade red blood cells. One common target is the glycophorin proteins. These proteins seem to normally serve as lubrication on the surface of red blood cells so they don’t get stuck in arteries and veins and cause strokes.
Three glycophorin proteins, A, B, and E are coded for by genes on chromosome 4 (Humans have 23 chromosome pairs). Glycophorin A and B are used in plasmodium invasion. Leffler and colleagues were able to show that DNA segments are deleted and duplicated in this region of chromosome 4 about ten times more frequently in the African samples they analyzed compared to samples from other continents.
They were able to characterize one particular alteration from East Africa that appears to reduce the probability of severe malaria by 40 percent. In this rearrangement, called DUP4 (duplication #4), the glycophorin E gene is duplicated, the glycophorin B gene is deleted, the glycophorin A survives unmolested, and two new Frankenstein-like genes are created which are fusions of glycophorins A and B.
This illustrates a common pattern in the evolution of genes. New genes are often made from the parts of old ones. This is one way evolution gets around the “complexity” problem: genes are usually not made from scratch. Also once a gene is duplicated each copy can follow its own path. It is worth noting that the study by Leffler and colleagues was made possible by the addition of additional samples from people of African descent.
Dispersing the Miasma
The treatment and prevention of malaria only made progress when the miasma theory was discarded. It is thought that some future anti-malarial drugs will attempt to disrupt the binding of Plasmodium to red blood cells. These strategies will have to be based on the thorough understanding of the relationship between Plasmodium and humans. The findings by Leffler and colleagues illustrates that this relationship is dynamic and subject to ongoing evolution and change.
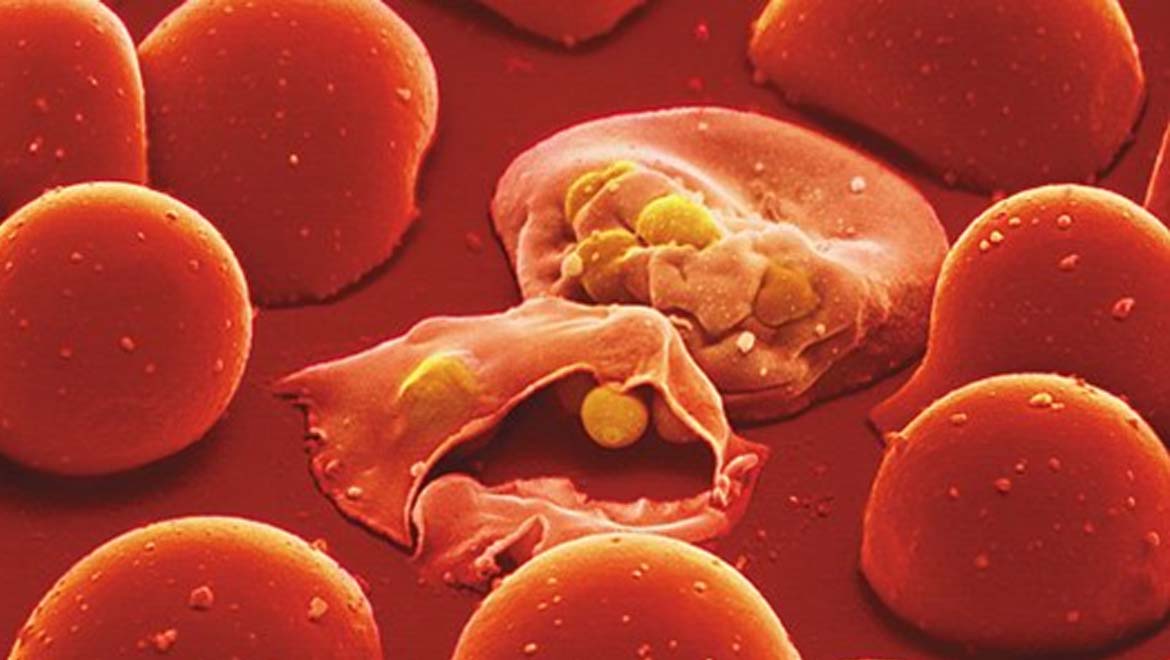
Top image: Malaria Parasite. (Emedmd)
References
Leffler, E. et al., 2017. Resistance to malaria through structural variation of red blood cell invasion receptors. Scince, 356(6343).
National Center of Biotechnology Information, 2017. GYPB glycophorin B (MNS blood group) [ Homo sapiens (human) ]. [Online]
Available at: https://www.ncbi.nlm.nih.gov/gene/2994#genomic-context
[Accessed 14 July 2017].
World Health Organization, 2011. Sickle-cell disease and other haemoglobin disorders. [Online]
Available at: http://www.who.int/mediacentre/factsheets/fs308/en/
[Accessed 14 July 2017].
World Health Organization, 2017. Malaria. [Online]
Available at: http://www.who.int/mediacentre/factsheets/fs094/en/
[Accessed 14 July 2017].




No comment