A recent paper in Nature has reported the first steps towards the technology to create embryos from cells that are not those of the egg or sperm variety.
This procedure has been done to demonstrate the biological cues by which single stem cells divide into those that ultimately form embryos and placentas. The results of this research can be termed as artificial blastocysts or ‘blastoids’, as the scientists referred to them as.
Blastoids mimic the physiology of the very early embryo, which could enable researchers, in the future, to study it in greater detail. This work could also enhance the study of fertility and offer a whole new, animal-cruelty-free methodology of drug development.
However, it appears that blastoids cannot result in viable pregnancies (not that this was the point of the study).
Exploring the Blastoid Technology
The discovery of the blastoid technology was documented in a paper published in the May issue of Nature. It was developed by scientists from the MERLN Institute for Technology-Inspired Regenerative Medicine, the Hubrecht Institute for Developmental Biology and Stem Cell Research and Utrecht University in the Netherlands.
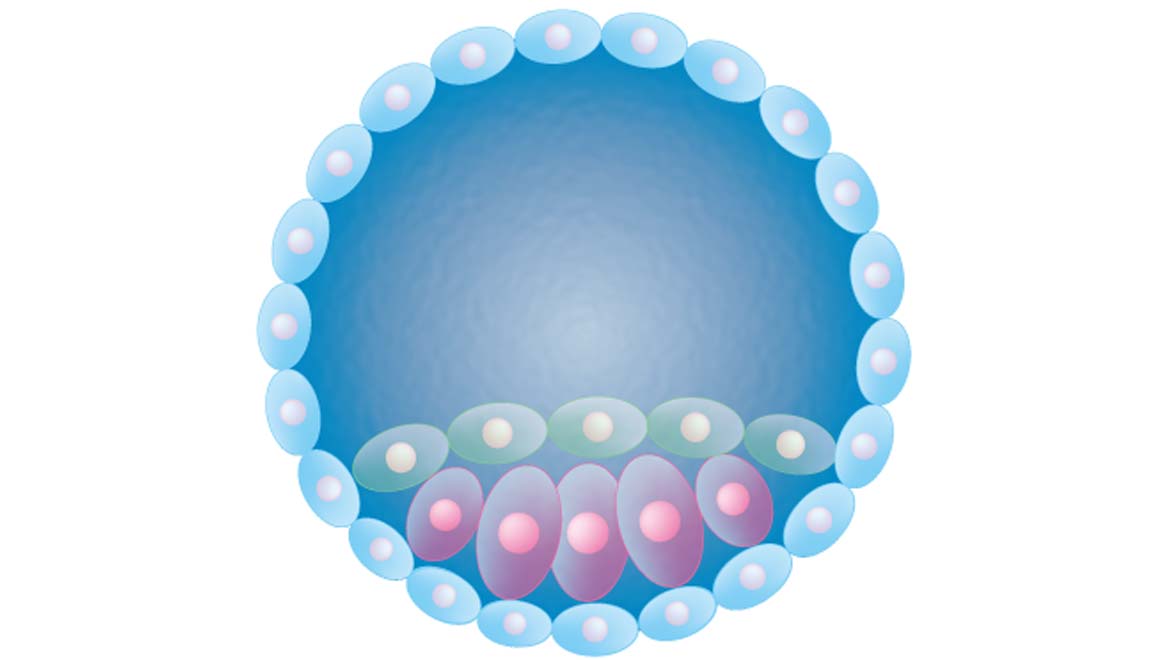
The article claims to demonstrate that embryonic formation can be tracked, via biochemical pathways, from a non-gamete stem cell. As a result, this cell would split into many others, which form a hollow sphere known as a blastocyst. This is a conventionally-accepted model of how embryos start out in humans and many other animals.
The blastocyst, formed of two basic layers, built the placenta (more or less on the 'outside' of the rapidly deforming sphere) and the embryo (makes up the off-center core of the mature blastocyst).
This technique was also performed in response to a sequence of biomolecular 'orders,' which mostly emanated from the proto-embryonic core, as research has found.
The Dutch team reported that their blastoids propagated this through a number of pathways the orders took, including the BMP4/Nodal-KLF6 hub of embryonic cellular activity. This mechanism is an example of a signaling axis that has been studied in more detail. However, others are not yet completely understood.
Therefore, the new possibilities implied by blastoid technology include the enhanced investigation and elucidation of blastocyst activity.
The blastoids were made by removing cells from pre-existing mouse blastocysts. This procedure was performed, by the team, at a stage in which the blastocysts can easily regenerate one of their own cells, if necessary.
The investigators found that isolating blastocyst cells from both the proto-embryonic and pre-placental variety could result in their rearrangement into a new blastoid, when cultured and treated correctly.
These cells were verified by assessment of the blastoid's genetic and morphological profiles, which reportedly matched those associated with a 3.5-day-old blastocyst.
The authors also reported that placing a blastoid into the womb of a mature mouse resulted in its implantation into the uterine wall as normal, due to the intact and functioning signaling framework.
However, the blastoids were not associated with the ability to develop into a more mature embryo, let alone a fetus. But, the new concept will be helpful in studying embryonic signals in greater detail.
The blastoids documented in this paper were all derived from mice, although, work from this study may lead to the generation of human versions in the future.
Future of the Blastoid Concept
This paper may be a valuable starting-point for improved fertility science and embryonic studies.
In addition, blastoid cells could also facilitate drug development that does not rely on animal models, as other stem cell types do today.
This study could offer concrete evidence that the proto-embryo part of the blastocyst directs the development of the placenta, and of itself, in utero, as a result of its automatic signaling mechanisms.
Also, blastoid cells typically retain the potentially desirable stem cell property of regeneration. This could be valuable to the regenerative medical science of the future.
This piece of research has demonstrated the ability to create, in the future, artificial embryos from stem cells, whose lines have been in existence for some time.
It is also a significant achievement of bioengineering to induce the same cells to form such a true, three-dimensional blastocyst analog. Although, this new technique has not yet been replicated in follow-up studies.
Presuming that it will be applied to science, this investigation could pave the way to the refinement and enhancement of many scientific disciplines, especially the studies of embryonic development and fertility.
Blastoid technology may, additionally, be useful in the development of other therapeutic areas like regenerative medicine.
Overall, it remains to be seen if this new Dutch research breakthrough will realize the potential it truly represents.
Top Image: Diagram showing a cross-section of a blastocyst. (Source: Database Center for Life Science (DBCLS))
References
N. C. Rivron, et al. (2018) Blastocyst-like structures generated solely from stem cells. Nature. 557:(7703). pp.106-111.
S. Wennekamp, et al. (2013) A self-organization framework for symmetry breaking in the mammalian embryo. Nat Rev Mol Cell Biol. 14:(7). pp.452-459.
S. E. Harrison, et al. (2017) Assembly of embryonic and extraembryonic stem cells to mimic embryogenesis in vitro. Science. 356:(6334).
Scientists Craft Embryo From Stem Cells Without Egg or Sperm, 2018, nexpected, https://www.nexpected.com/2018/05/scientists-craft-embryo-from-stem-cells.html , (accessed 6 May 2018)




No comment