CRISPR/Cas9 has become the most prominent form of potential gene therapy for various conditions.
However, the concept is still associated with issues that need to be addressed, before it can be applied to conventional medical settings.
For example, CRISPR/Cas9 is a molecular 'machine' for gene-editing, which may be delivered into the body using viruses. These vehicles, despite being harmless, are often targeted by the human immune system and wiped out. The elimination prevents future propagation of the desired gene-editing, and thus, the end of (hypothetical) treatment efficiency. Even if the viruses survive, the genes that are edited may re-assert their normal/disease-causing states, over time.
Safe Havens in the Genome
There is a potential solution for these types of CRISPR failures - the use of a 'safe haven' locus (a certain spot on the genome that does not affect the appearance or function of any cell type, if it is altered in any way).
ROSA26 locus has gained quite some popularity in animal models of CRISPR. It is present in a variety of cell types and driven by the promoter for the ROSA26 gene.
Safe havens such as ROSA26 are used to 'add' a corrective gene using CRISPR, as part of a technique known as 'knock-in' gene editing. This insertion of an additional gene (s) does not disrupt normal health, even though the knocked-in sequence may be expressed in considerable numbers over time.
Knock-ins using safe havens are also superior to other techniques as they result in the expression of only one copy of the corrective gene.
Alternative strategies, such as the insertion of a gene next to another biologically relevant sequence, may result in adverse events such as the duplication or disruption of the desired gene. This activity may, in turn, result in less than predictable or reliable expression patterns for the gene, or even, the complete loss of its expression.
There is, also, a risk that the insertion of one active gene into another may result in a new oncogenic profile in the locus. Therefore, the ROSA26 locus is being used in a number of animal models for new CRISPR-driven treatments. One of these studies includes a potential treatment for serum deficiency-related conditions.
Gene Therapy for Serum Deficiency
Serum deficiency is a potentially deleterious health state associated with hereditary mutations in specific genes. The addition of relevant corrective genes may enhance serum production in a patient, thus alleviating the condition.
A team of researchers set out to model such a strategy in mice using a combination of adenovirus-mediated CRISPR and ROSA26 localization. These scientists, who completed their project at Washington University's (Saint Louis) School of Medicine, proposed that this combination might result in long-lasting expression of their two chosen corrective genes: human EGFP and AAT.
A type5 adeno-associated virus vector was chosen for the experiment, as it is known for reproducible efficiency in delivering CRISPR-Cas9 machines. They were loaded with all the genes necessary to produce CRISPR products that specifically targeted the ROSA26 locus in the livers of the experimental C57Bl/6J mice.
The process was conducted to assess the concentrations of human AAT and EGFP proteins in these organs, over time. The group found that the genes were ‘knocked-in’ successfully through gene- and protein-detection studies (e.g. PCR and Western Blot).
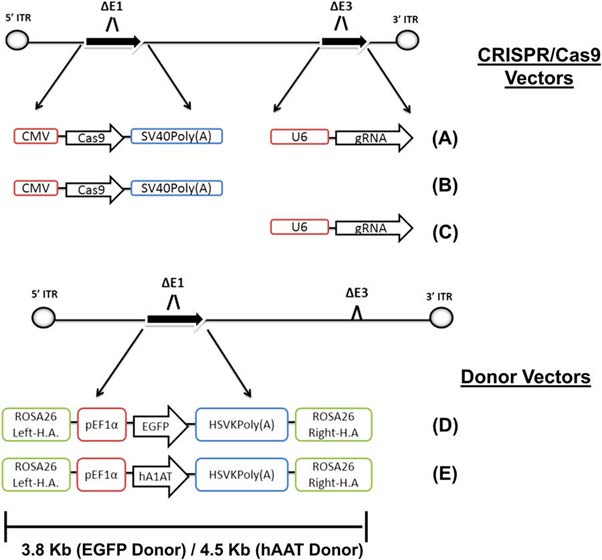
The genetic contents of the adenoviral vector. (Source: Stephens CJ et al., 2018)
The team reported that the expression of human AAT, as a result of adenovirus-delivered CRISPR in mouse liver samples, persisted for 200 days after initial inoculation.
The presence of the new gene in the ROSA26 locus was confirmed using PCR, as was its inheritance by the daughter cells of the originally-targeted hepatic cells.
On the other hand, the EGFP gene persisted in the same manner for only 36 days. The investigators noticed that some of the mice exhibited a significant reduction in the concentration of the adenovirus-associated genes. This decrease was thought to be related to the high cytotoxicity and immune response caused by Cas9, a large gene-cleaving enzyme derived from bacteria. Nevertheless, the AAT expression, more or less, remained constant across the mice.
The researchers concluded that, based on the longevity of human AAT expression and rate of gene delivery into the ROSA26 locus (calculated at approx. 13.5% overall), the project was – a success!
The ability of virus-mediated CRISPR to produce a 'knock-in' of a supplementary gene in a serum deficiency model was demonstrated.
The team also reported that the treatment resulted in a low incidence of adverse events and negligible off-target delivery, where the gene is added to another locus elsewhere in the genome.
Therefore, future studies and development of this form of CRISPR strategy using other serum-related genes can be explored.
Conclusion
A recent study, published in the issue of Nature Gene Therapy, has demonstrated that CRISPR can be a long-lasting, effective therapy in adenovirus-assisted delivery, using loci such as ROSA26.
But previous research has shown that the ROSA26 expression rate is moderate, at best. The issue may necessitate a lengthy process of refinement before this form of CRISPR can be approved for use in human patients.
Top Image: A colored, enhanced representation of an adenovirus. (Source: Public Domain)
References
Stephens CJ, Kashentseva E, Everett W, Kaliberova L, Curiel DT. Targeted in vivo knock-in of human alpha-1-antitrypsin cDNA using adenoviral delivery of CRISPR/Cas9. Gene Therapy. 2018.
Tchorz JS, Suply T, Ksiazek I, Giachino C, Cloetta D, Danzer CP, et al. A modified RMCE-compatible Rosa26 locus for the expression of transgenes from exogenous promoters. PLoS One. 2012;7(1):e30011.
Evangelou J. CRISPR enhances gene therapy to fight inherited diseases. 2018. Medical Xpress. Available at: https://medicalxpress.com/news/2018-04-crispr-gene-therapy-inherited-diseases.html





No comment