Today, there are many projects that investigate new and emerging treatments that address a critical factor in cancer progression: the tumor microenvironment. This is a complex process, in which cancer cells adjust the conditions within, and also possibly in the immediate vicinity of, the tumor they have formed to their own ‘liking’. Tumors develop their own internal environments for a number of reasons, which include resistance to aspects of the patient’s immune system which could be capable of destroying or damaging them otherwise. The tumor microenvironment, as these ‘custom’ set of conditions is known, tends to be low on oxygen, more acidic, high in lactate (a waste product of many cell types) and deprived of glucose. This is typically contrary to the conditions needed for healthy, normal tissues. Therefore, part of the function of the tumor microenvironment (TME) may be to help it invade new tissues by damaging their health and depriving them of nutrients. In addition, these TME conditions also tend to enhance resistance to drugs developed to kill cancer cells.
As a result of this, many modern cancer treatments are directed at boosting anti-tumor immune responses or disrupting TMEs in other ways. This includes photodynamic therapy (PDT; in which the tumor is directly subjected to a light source to kill cancer cells and increase oxygen levels within the TME). However, they are sometimes less effective than doctors and scientists may wish, due to the adaptability and resilience of the tumors in question. As a result, researchers have turned to nanotechnology as a way of improving the delivery of destructive or immune-modulating therapies into tumors. Nanotechnology may also enhance the accuracy with which clinicians target cancerous tissues.
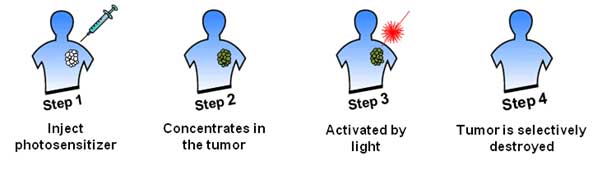
Illustration of Photodynamic Therapy (PDT) (photolitec.org)
Nanotechnology and cancer
An increasingly effective example of nanotechnology in cancer treatment is the use of manganese oxide (MnO2). These MnO2 particles have demonstrated the ability to increase oxygen levels in TMEs by reacting with the hydrogen peroxide that is often found within tumors. In addition, if MnO2 reacts with TME molecules that increase pH levels, they increase the visibility of tumors under T1-weighted magnetic resonance imaging (T1-MRI). Therefore, many researchers see the potential of arranging MnO2 into structures at the atomic level that can carry molecules of anti-cancer drugs into tumors and release them on contact with acidic TME chemicals. This strategy has been shown to enhance some types of radiotherapy and PDT in previous studies. In addition, MnO2 is broken down into harmless manganese ions in the body and expelled by the kidneys; in fact, manganese is a mineral nutrient, if required in tiny doses only by humans. Therefore, MnO2 nanostructures should be completely non-toxic, as opposed to some other nano-drug delivery systems.
One of the best-known nanostructures for drug delivery are hollow, partially porous (i.e. having holes in their surfaces) shells that can carry a relatively large dose to its target location. However, there is not much progress on the formation of MnO2 into a hollow nanoparticle for anti-tumor applications. A team from the Institute of Functional Nano & Soft Materials (FUNSOM) and the Jiangsu Key Laboratory for Carbon-Based Functional Materials & Devices at Soochow University in China decided to address this by depositing MnO2 onto spherical silica nanoparticles which were then dissolved away to leave hollow manganese shells. These shells were ‘filled’ with the chemotherapy drug doxorubicin, the PDT-enhancing compound chlorine-e6 (Ce6) or both to test them on experimentally-induced murine tumors.
The team found that treating cultured cancer cells with MnO2 shells loaded with Ce6 was considerably more effective in causing cell death that treatment with Ce6 alone, albeit only when the concentration of Ce6 was higher (e.g. at 5µM compared to 0.625µM). In vitro studies also showed that the nanoshells also distributed both doxorubicin and Ce6 within the cells effectively. However, the full treatment, which included administering shells containing both drugs, did not kill the cells effectively unless it was also combined with PDT (which entailed exposure to radiation at a wavelength of 660nm in this study). In this case, it reduced the cell population to between 80 percent and 20 percent, which was again dependent on the increase in dose for both drugs.
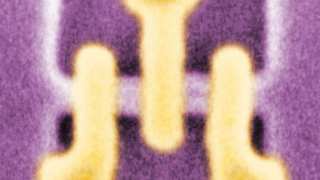
The team then moved on to treating experimental tumors in live animals with the nanoshells with and without the drugs. They found that tumors treated with the nanoshells significantly increased their visibility when imaged using T1-MRI, compared to untreated tumors and to normal muscle tissue into which the nanoshells were also introduced. Tumors treated with the nanoshells containing both drugs exhibited a marked loss of volume over 14 days, although the addition of PDT again resulted in a significantly greater effect compared to nanoshell-delivered drug therapy alone.
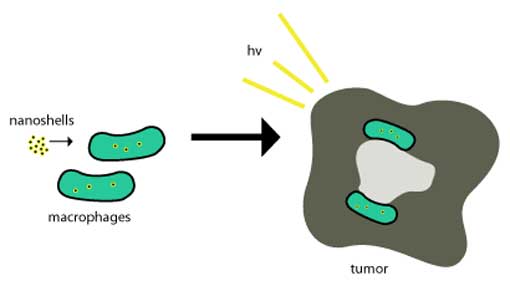
Nanoshells taken into tumors. (Public Domain)
The nanoshell/drug treatment also improved immune responses to tumors. For example, it enhanced the infiltration of cytotoxic T lymphocytes (CTL), which normally destroy abnormal cells, into tumors, as well as concentrations of an ‘anti-tumor’ cytokine, IFN-gamma. The team then decided to add another emerging form of cancer treatment known as PD-L1 blockade. This therapy is based on observations that tumors ‘hijack’ and over-express PD-L1, a physiological regulator intended to ‘de-commission’ CTLs in tissues that have already been cleared due to their actions and those of other similar immune-system cells. This protects tumors from destruction by CTLs under normal circumstances. Therefore, the FDA has recently approved drugs such as atezolizumab, which inhibit PD-L1. The addition of an anti-PD-L1 drug to the nanoshell-delivered drug regimen also significantly reduced tumor mass and volume over time, while again enhancing CTL invasion and other anti-tumor immune functions.
Potential for the future
The team from Soochow University have demonstrated that hollow shells of nanoscopic manganese oxide may form a drug-delivery system that may have particular potential when introduced into the TME. Their study, as described in a paper published in Nature Communications, may demonstrate the ability of MnO2 nanoshells to enhance PDT combined with chlorine-e6. This treatment, alone or in combination with PD-L1 blockade therapy, may also improve the immune response to the presence of a tumor, which the tumor typically defends against by exploiting and exaggerating T-cell regulation. The researchers who completed this study attribute most of the efficacy apparently demonstrated by the nanoshells to their ability to increase oxygen in the TME. On the other hand, this is a preliminary study of hollow manganese nanostructures in cancer treatment, and requires further validation and development. However, it does demonstrate that MnO2 may have much more of a role in the future of cancer treatment and imaging.
Top image: Atypical carcinoid tumor (CC BY-SA 2.0)
References:
Yang G, Xu L, Chao Y, Xu J, Sun X, Wu Y, et al. (2017) Hollow MnO2 as a tumor-microenvironment-responsive biodegradable nano-platform for combination therapy favoring antitumor immune responses. Nature Communications. 8:(1). pp.902.
Miao L, Wang Y, Huang L. Tumor microenvironment and nanodrug delivery. Nanomedicine: Nanotechnology, Biology and Medicine. 12:(2). pp.452.
Pardoll DM. (2012) The blockade of immune checkpoints in cancer immunotherapy. Nature reviews Cancer. 12:(4). pp.252-64.



No comment