Clinical trials and their results affect us all. These trials can dictate the level of innovation and improvements in the healthcare sector that is available to us.
Furthermore, depending on location, the resources and funds used in clinical trials may come from individuals’ tax contributions.
In addition, people with certain conditions may be strongly motivated to participate in these trials. These patients may do so to help beat a disease or, at least, alleviate the condition for themselves and others, in the future.
Therefore, the public has a right to know that these trials are being run properly and how they pan out, ultimately. This is particularly important as the clinical trial process is a necessary component of bringing new therapeutic options to the market. The invigilators will also be held to, appreciably, high regulatory and ethical standards.
Ensuring Integrity of Clinical Trials
Unfortunately, some healthcare researchers and experts have been suggesting lapses in these expectations, on the part of some clinical trial investigators, in recent years. In extreme cases, this has led to the mysterious disappearance of the final trial reports at designated end-points.
The most recent review on this subject reported that only 50% of all trials registered with authorities such as the United States’ Food and Drug Administration (FDA) make their results available.
Registration with bodies such as the FDA is a mandatory step in gaining approval to run a clinical trial. Therefore, it is astounding that so many trials fail to report their results.
This information may be of crucial importance to many stakeholders, including patients, in the development of treatment plans.
However, making results available is not a formal legal requirement in the US or EU. This is subject to change in the US, however, with the advent of the FDA's Amendments Act of 2007 (FDAAA 2007). Under this act, all trials registered on or after January 1st, 2018, will be under more emphatic legal requirements to release their results.
But some healthcare experts are prepared to be less than complacent while waiting for these new set of rules to come into place.
Guidelines for Reporting Results
The FDAAA 2007, also known as Final Rule 42 CFR Part 11, specifies that all trials with an initiation date of 1 January 2018, or with a completion date in January 2008 or later, must report their results.
The definition of the term 'trial' is broad, as it covers all applicable or possibly applicable trials evaluating a potential drug, device, genetic product, radiotherapy, biologic therapy, vaccine, diagnostic method, or a combination of any of these.
However, there have known to be a few loopholes in this law that may allow the evasion of this requirement. One of them includes the failure to declare a valid US location in a trial's registration. Also, a trial may not be of the first phase in the overall clinical trial process or one that tests the feasibility of a device.
FDAAA Trials Tracker: New Clinical Trials Reporting Tool
Ben Goldacre is a healthcare researcher and writer from Oxford University with a long-standing interest in adequate clinical trial reporting.
Along with his colleagues, Seb Bacon and Nicholas P. DeVito, Goldacre set out to track the actual compliance to FDAAA 2007, and assess the readiness to report to current and recent investigators.
The team, thus, developed an informatics toolset to compile a database of all clinical trials that report in accordance with the Act, if they are eligible. This tool is known as the FDAAA Trials Tracker.
The FDAAA Trials Tracker incorporates data on all trials that are applicable or possibly applicable under the Act, as well as their given sponsors and completion dates. This information will be sent to a live-tracking website, which will also be updated with alerts when a trial releases its final data.
Goldacre and his colleagues argue that tools such as these are necessary to enforce the consequences (legal or otherwise) for the non-reporting of clinical data. A new system of fines, which can be levied for late submissions, under FDAAA 2007, has been included.
The Trials Tracker is available at fdaaa.trialstracker.net.
Users can view or download the information the website has gathered, to date, which is also updated regularly.
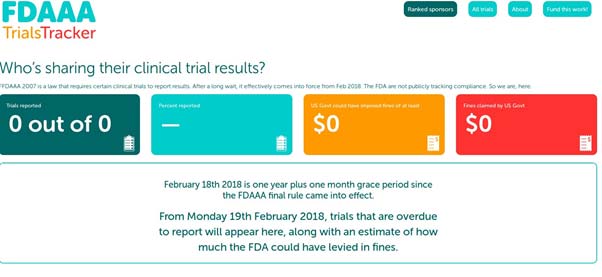
Sample report from the FDAAA Trials Tracker website. (Source: DeVito, N. J. et al., 2018)
The data that makes up the Trial Tracker is derived from XML files from clinicaltrials.gov, on which all trials seeking FDA approval are obliged to register.
Investigators, additionally, need to post all applicable data on the nature, aims, participants, and outcomes of their trial on this website. This could, possibly, make it an authentic and timely source of result submission.
Goldacre believes that a tool like Trial Tracker will make the documentation of actual submissions clearer, more straightforward and more user-friendly.
The website also facilitates the calculation of the system of fines that can be levied for late reporting under FDAAA 2007.
The Trials Tracker was compiled using a database template from Google's BigQuery analytics platform and open-access code licensed from MIT through a public GitHub.
The tool may also be joined by analogs that monitor the same compliance in the context of the European Medicines Agency and specific therapeutic areas.
The researchers of the study hope that the website will help reporting within the clinical trial process - a more open, transparent and compliant system with applicable legislation.
Top Image: The inappropriate reporting of clinical trials in the past has led researchers to build a tool called FDAAA Trials Tracker, which keeps a check on trials that are noncompliant. (Source: Pixabay).
References
N. J. DeVito, et al. (2018) FDAAA TrialsTracker: A live informatics tool to monitor compliance with FDA requirements to report clinical trial results. bioRxiv.
C. Schmucker, et al. (2014) Extent of Non-Publication in Cohorts of Studies Approved by Research Ethics Committees or Included in Trial Registries. PLoS One. 9:(12). pp.e114023.
Who’s sharing their clinical trial results?, 2018, fdaaa.trialstracker.net, https://fdaaa.trialstracker.net/ (accessed 24 May 2018)






No comment