Royal jelly is of potential interest to researchers in the areas of regenerative medicine and science. This compound is secreted by honey bees (Apis mellifera) and functions to transform ordinary bee larvae into queen bees. This process is most likely done by certain molecules in the jelly, which is known to act in an epigenetic manner.
We know this because queens are essentially genetically identical to ordinary worker bees. However, they have key differences; for example, the queen grows many more cells compared to the average subject and is capable of regeneration where all the other bees in a hive are not.
Royal Jelly and Regenerative Medicine
Studies on royal jelly and its functions have also found that this product has specific effects on stem cells - one of them is the jelly promotes the replication of cells, in vivo, which has positive knock-on effects on cellular regeneration and healing in many tissues. This has been demonstrated in species besides bees such as several mammalian species.
Therefore, it seems that royal jelly or rather, the protein responsible for its actions may have a role in the future of medicine for humans.
Scientists have isolated this protein and characterized it as major royal jelly protein 1 (MRJP1), also known as royalactin. It has, for example, been shown to stimulate proliferation and healing-like functions in cultured mouse liver cells.
However, there is still much to learn about royalactin. For instance, it has not been clear how the protein promotes cellular growth and division, as seen in prior research.
A group of scientists from the Stanford University School of Medicine, in collaboration with others from the Veterans Affairs Palo Alto Healthcare System, California’s LakePharma, Inc., and Minnesota’s R&D Systems, Inc., Minneapolis, hypothesized that the royal jelly protein may function as the known-drivers of ‘stem-celled-ness’ (or pluripotency) in an animal model.
These transcription factors promote division in stem cells, which in turn support the near-logarithmic proliferation seen in embryonic tissues. It could also boost a complete regeneration in damaged or absent tissue. Normally, these processes are inhibited or impeded in humans by processes such as scar formation and stem cell depletion.
Self-replicating stem cells are produced for laboratory purposes by inhibiting cellular MAPK/ERK Kinase (Mek) and glycogen synthase kinase-3 (GSK3) through the administration of proteins such as leukemia inhibitory factor (LIF) in otherwise protein-free media. This results in the establishment of a germline culture that resembles the embryonic stem cells of the species in question.
However, this procedure has some flaws: protracted Mek inhibition leads to genetic and epigenetic errors over time, thus degrading the stem cell line, over time, until they are eventually unusable. For example, stem cells, grown in LIF-enriched medium (medium/LIF+) that are subsequently placed in normal, serum-containing media without LIF (serum/LIF-), start to differentiate into ‘normal’ cells. They will then never revert back to the stem cell state, even if LIF is added back into the medium again (or is placed in serum/LIF+).
Royalactin vs. Leukemia Inhibiting Factor (LIF)
The discovery of improved stem cell promoters would be highly desirable to those in the relevant fields. It seemed to the Stanford team that royalactin could be the factor these scientists were after.
Therefore, they set up an experiment in which mouse stem cell lines were established in the normal way, differentiated using (serum/LIF-) medium and then placed in (serum/LIF+) medium to demonstrate the loss of pluripotency. This was a control for identical stem cells that were also differentiated but then placed in (serum/royalactin+/LIF-) medium. These cells exhibited a dose-dependent return to a stem-cell form over several passages (or transfers to fresh (serum/royalactin+/LIF-) medium).
In other words, it appeared that the royalactin restored the appearance and behavior of stem cells to these cells in culture, in a way that LIF could not.
The team conducted further analysis of these cells and found that their genetic profile had a “high degree of similarity relative to those cultured in the presence of LIF.”
They also found that the royalactin treatment appeared to result in the establishment of specific patterns of gene expression necessary to maintain a pluripotent state, e.g., the Rex1 marker and a LIF-independent pattern of Stat3 inhibition.
The scientists ‘transplanted’ royalactin-generated stem cells into mouse blastocysts (a common stem cell science technique known as chimerization), which resulted in the development of normal, healthy live animals whose stem cells persisted in exhibiting the royalactin-associated genetic profile.
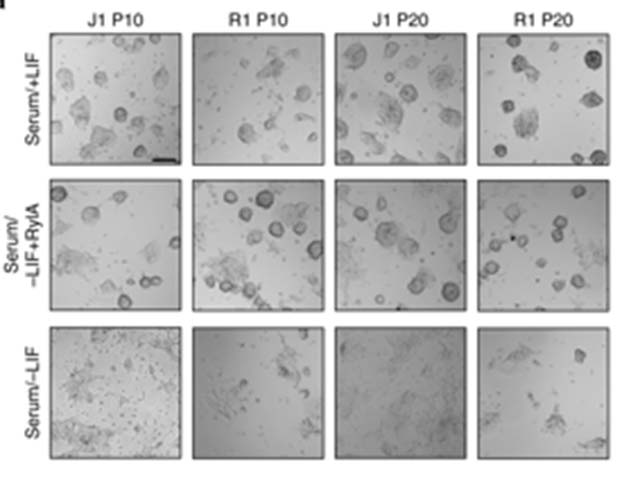
The morphological analysis of serum/LIF+ treated stem cells, compared with serum/royalactin+ cells and stem cells placed in serum/LIF- medium. The latter have differentiated, whereas the other two groups have retained the basic, round stem-cell shape. (Source: D.C. Wan et al, 2018)
Is There a Royalactin for Mammals?
The group of researchers analyzed the protein structure of the royalactin protein in detail and found it to have six specific domains that resembled the wing-blades of a drone in motion, which are, in fact, called beta-propeller domains. The structure was compared against numerous protein-expression databases using iterative PSI-BLAST for a mammalian homolog.
This resulted in the identification of a recently-discovered mouse protein NHL Repeat Containing 3 (NHLRC3). It also had six propeller domains and resembled royalactin. Not much is known about NHLRC3 to date, besides the fact that it begins to be expressed after the first few days of murine embryonic development.
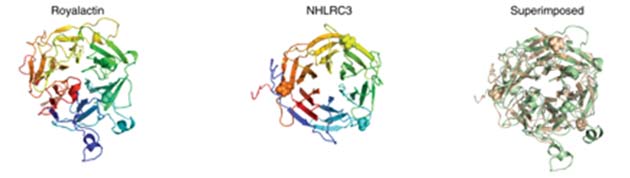
The structure of royalactin compared to that of NHLRC3. (Source: D.C. Wan et al, 2018)
The scientists found that the placement of stem cells in (serum/LIF–/NHLRC3+) medium gave the same results as the (serum/royalactin+/LIF-) procedure. In addition, chimerization using these stem cells again resulted in the generation of healthy animals with the expected genetic profile. Therefore, the team concluded that NHLRC3 was most likely a heretofore unknown mediator of pluripotency.
Their paper written on this subject, and published in the journal, Nature Communications, indicates that it may be a viable candidate for the promotion of ‘stem-ness’ in the lab that may be a superior option to LIF. This, in turn, could lead to the development of a more sophisticated, effective regenerative medical science to benefit the humans of the future.
Top Image: Natural royal jelly protein secreted by honeybees. (Source: Skincare.org)
References
D. C. Wan, et al. (2018) Honey bee Royalactin unlocks conserved pluripotency pathway in mammals. Nature Communications. 9:(1). pp.5078.
M. Li, et al. (2017) Ground rules of the pluripotency gene regulatory network. Nature Reviews Genetics. 18: pp.180.
A. G. Smith, et al. (1987) Buffalo rat liver cells produce a diffusible activity which inhibits the differentiation of murine embryonal carcinoma and embryonic stem cells. Developmental Biology. 121:(1). pp.1-9.
H. Bernien, et al. (2017) Probing many-body dynamics on a 51-atom quantum simulator. Nature. 551:(7682). pp.579-584.
J. Majtan, et al. (2010) Effect of honey and its major royal jelly protein 1 on cytokine and MMP-9 mRNA transcripts in human keratinocytes. Experimental Dermatology. 19:(8). pp.e73-e79.
C. K.-M. Chen, et al. (2011) The many blades of the β-propeller proteins: conserved but versatile. Trends in biochemical sciences. 36:(10). pp.553-561.





No comment